Development and validation of prediction score for post-tuberculosis obstructive lung disease
Pefura-Yone Eric Walter 1,2 , Balkissou Adamou Dodo 3 , Djenabou Amadou 4 , Poka-Mayap Virginie 2 , Moifo Boniface 5 , Kouna-Abene Francine Gaëlle 6 , Tanyi Brenda 6 , Youmbi Christelle Fallone 7 , Kuaban Christopher 8 , Kengne André Pascal 9
1 Département de médecine interne, Faculté de médecine et des sciences biomédicales, Université de Yaoundé 1, Yaoundé, Cameroun
2 Service Pneumologie A, Hôpital Yaoundé Jamot, Yaoundé, Cameroun
3 Faculté de médecine et des sciences biomédicales de Garoua, Université de Ngaoundéré, Garoua, Cameroun
4 Centre de traitement approuvé pour le VIH, Hôpital Yaoundé Jamot, Yaoundé, Cameroun
5 Département de radiologie et d’imagerie médicale, Faculté de médecine et des sciences biomédicales, Université de Yaoundé 1, Yaoundé, Cameroun
6 Faculté de médecine et des sciences biomédicales, Université de Yaoundé 1, Yaoundé, Cameroun
7 Institut Supérieur de Technologie Médicale, Yaoundé, Cameroun
8 Faculté des sciences de la santé, Université de Bamenda, Bambili, Cameroun
9 Medical Research Council, Le Cap, Afrique du Sud
TO CITE: Pefura-Yone EW et al. Development and validation of prediction score for post-tuberculosis obstructive lung disease. The Papers of Medical Sciences 2019;1:e002.
KEYS WORDS: obstructive lung disease, COPD, tuberculosis, prediction score, risk factors
ARTICLE INFO
Received: 11th February 2019 Accepted: 13th March 2019 Available online: 14th March 2019
Correspondence to: Pefura-Yone EW, Email: pefura2002@yahoo.fr
ISSN: 2663-7545
Copyright ©2019, Pefura-Yone et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original authors and source are credited.
ABSTRACT
Objective: To derive and validate a prediction score for the presence of post-tuberculosis obstructive lung disease (post-TB OLD) in adult patients at the end of PTB.
Methods: We used data of patients aged ≥18 years successfully treated for PTB, at the Yaounde Jamot Hospital, Cameroon from January 2015 to May 2017. A simple prediction score was constructed using regression coefficients for predictors in the final model. The model’s discrimination was assessed using c-statistics and calibration assessed via Hosmer and Lemeshow (H-L) statistics. Internal validation used bootstrap resampling procedures.
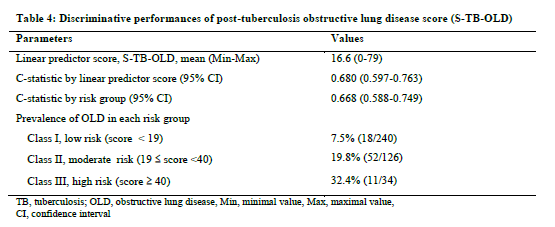
Results: A total of 400 patients (men 53.5%) with a median age (25th -75th) of 38(29-49) years were included. Fifty four patients had post-TB OLD, giving the prevalence (95% CI) of post-TB OLD of 13.5%. (10.3-17%). The independent determinants of post-TB OLD were proportion (%) of lung sequelae [odds ratio (95% CI): 1.04(1.02-1.06) per 1% increment, β = 0.042] and smoking [2.19(1.18-4.08), β =0.783]. The resulting score for prediction of post-TB OLD (S-TB-OLD) was therefore expressed as: S-TB-OLD= Proportion of lung sequelae (%) + 19 (if tobacco smoking). The c-statistic was 0.680 (95% CI: 0.597 – 0.763). At the optimal cut-off point score of 19.31, the sensitivity, specificity and negative predicted value were 67%, 65% and 92.5%, respectively. The calibration was good [H-Lχ2= 8.135 (p = 0.321)] and the optimism was marginal in boostrap resampling.
Conclusion: Confirmation of the usefulness of this score should help to stratify patients according to the risk of developing post-tuberculous obstructive post-TB OLD in order to improve their management.
INTRODUCTION
Tuberculosis (TB) remains a major public health problem in spite of existing prevention and control strategies. The World Health Organization (WHO) 2018 global TB report estimated a total of 10 million incident TB cases worldwide in 2017, with a death toll of 1.6 million people(1). In Cameroon, approximately 26,000 TB cases were notified in 2017(2). Even after a well conducted treatment, pulmonary tuberculosis (PTB) heals leaving pleuro-parenchymal sequelae of variable degrees due to its fibrogenic character. Chest X-Ray (CXR) is the most used imagery exam in the detection and follow-up of PTB sequelae. The prevalence of CXR sequelae at the end of a TB treatment is elevated, varying between 66.2 to 88%(3–6). These sequelae are dominated by residual cavities and fibro-nodular opacities(3). These pleuro-parenchymal sequelae can have a permanent repercussion on lung function, altering the quality of life of patients. Lung function abnormalities observed in sequelae phase are variable, with certain authors revealing a predominance of obstructive disorders; and with others, a predominance of restrictive disorders or mixed disorders(7–10). In recent studies published from 2011 to 2016 the prevalence of post-TB OLD ranges from 13.2% to 57.9%(7,9–12). In these recent studies, risk factors for post-TB OLD are not reported. Few studies have reported risk factors for post-TB OLD. In studies published prior to the 1990s, the risk factors for post-TB OLD were radiographic extension of lesions, duration of disease before TB treatment, age and tobacco smoking (13,14). In limited resources setting where tuberculosis predominates, it is important to stratify patients according to the risk of developing post-tuberculosis lung function impairment in order to optimize their management. Indeed, the aim of this study is to investigate the associated factors of post-TB OLD and generate a simple numerical score for predicting post-TB OLD in adults.
MATERIALS AND METHODS
Setting and participants
This study used data from three cross-sectional studies conducted from January to July 2015 (7 months), from December 2015 to May 2016 (6 months) and from January to May 2017 (5 months) at the Yaounde Jamot Hospital (YJH). YJH is the reference center for the management of TB and respiratory diseases for Yaounde (capital city of Cameroon) and its surroundings. The management of TB in this center is based on the international recommendations(15). The data were collected using non probabilistic, consecutive and exhaustive sampling method.
The adults’ patients successful treated for bacteriologically proven pulmonary TB at the Diagnosis and Treatment Center (DTC) of YJH were included. Patients with the following criteria were excluded: acute respiratory infection, chronic obstructive airway disease, asthma, interstitial lung disease, lung cancer and lung resection, congestive heart failure; contraindications to pulmonary function test (recent myocardial infarction, pneumothorax, pregnancy at third trimester, recent thoracic or abdominal surgery, a seriously altered general state or severe dyspnea limiting the correct execution of the spirometry maneuvers); inappropriate spirometry maneuvers. Ethical clearances were obtained from the Institutional Ethics Committee of the Faculty of Medicine and Biomedical Sciences of the University of Yaounde I (Data collected from January to July 2015, and from January to May 2017) and from Institutional Ethics Committee of Faculty of Medicine and Pharmaceutical Sciences of University of Douala (Data collected from December 2015 to May 2016). A research authorization was equally obtained from the administrative authorities of the YJH.
Data collection
Baseline data
The following social and demographic data were collected: age, sex, marital status, place of residence (urban, semi-urban and rural), higher level of formal education. Past medical history included HIV status; history of respiratory diseases such as pneumonia, pulmonary TB; other comorbidities influencing the respiratory function such as diabetes mellitus and heart failure. Tobacco smoking was evaluated in pack-years. Smoking habits were divided in following groups: former smokers (stopped smoking for more than 6 months at least), never-smokers; active smokers. Concerning alcohol consumption, patients having stopped drinking for at least 12 months were considered as former drinkers and occasional drinkers (alcohol use less than a day/week), regular drinkers and non-drinkers were also distinguished. Exposure to biomass fuel was evaluated by the type of fuel or combustible used to cook food in household.
Clinical data comprising duration of symptoms prior to tuberculosis treatment and persistence of respiratory symptoms at the end of TB treatment (cough, expectoration, and dyspnea) were collected. The dyspnea was graded according to modified Medical Research Council Scale (16). The weight was taken in kilograms with the aid of a CAMRY-branded scale (CAMRY, Guangzhou, China). The patient was dressed only in light clothing. The height was measured with a measuring gauge to the nearest cm in a patient standing straight up, with heels joined together and arms along the body. Body mass index in kg/m2 was calculated as the ratio of weight (kg) to height squared (squared meter).
Standard full-size postero-anterior chest X-ray (CXR) was used to quantify the extent of lung remodeling in patients. The nature, distribution, and extent of abnormalities were noted for initial and final CXR. Type of CXR lesions collected include: cavities, infiltrates, nodules, fibrotic lesions, miliary lesions, patchy or confluent consolidation and associated pleural effusion. Extension of radiographic lesions was appreciated by using total percentage of lung affected by any pathology on the initial and final chest X-ray (at the end of treatment). To grade the percentage of affected lung, visual estimation of the extent of opacification, cavitation or other pathology as a percentage of visible lungs was made. To evaluate this, each lung field was subdivided into three zones (upper, middle and lower zone) according to the method described by Ralph et al (17). The delimitation was done by two horizontal lines: the first is the horizontal line passing through the upper border of the chondro-costal junction of the anterior arch of the second rib to the lateral thoracic wall; the second is the horizontal line passing through the upper border of the chondro-costal junction of the anterior arch of the 4th rib to the lateral thoracic wall. Dense opacification of an entire zone was graded as 100% of that zone, while patchy consolidation within a zone attracted scores <100% depending on the extent of consolidation. An average percentage of lesions per lung field was then evaluated and an average of the two lung fields deduced to determine the total percentage of affected lung (% of pulmonary tuberculosis lung sequelae).
Spirometric data
Spirometry was performed using a turbine pneumotachograph (spiro USB, care fusion, Yorba Linda-USA) respecting the American Thoracic Society (ATS) 1994 standards(18). The exam was done by pulmonary function test technicians or by well-trained final year medical student.
All measurements were performed after at least 15 min rest, with the participant in a seated position, the back straight up, and nose clipped to allow air flow only by mouth. The American Thoracic Society/European Respiratory Society (ATS/ERS) acceptability and reproducibility criteria were applied (18). Instructions were explained before every maneuver; at least three tests were done per participant with the maximum being eight tests, while observing a rest period of at least one minute between consecutive tests to establish the forced vital capacity (FVC) curve. Spirometry variables measured included: forced expiratory volume in 1s (FEV1), forced vital capacity (FVC) and the FEV1/FVC ratio.
FEV1 and FVC values retained were the best out of the three tests which fulfilled the acceptability criteria (maximal difference below 5 % or 150 ml). All these measurements were expressed in absolute values and as a percentage of predicted value. Predicted values were estimated using Global Lung Initiative (GLI) 2012 references value (19). Quality control was done by regularly supervising investigator who performed spirometry. Spirometric curves were reviewed weekly by one of the experienced chest physicians working in YJH and feedback was made to investigators.
Data analysis
The data analysis used IBM-SPSS software for Windows version 23 (IBM, Chicago, US). Categorical variables were expressed as counts and proportions. Quantitative variables were summarized in terms of mean (standard deviation) when the distribution was considered normal; otherwise they were expressed in terms of median (25th -75th percentiles). The groups comparison used Chi-2 or Fisher exact test for proportions and Student t-test or non-parametric equivalent for continuous variables (Mann-Whitney U test or median test). Logistic regressions models were used to select independent determinants of post-TB OLD. Candidate variables were first tested for inclusion in univariable models, then the significant ones based on a p-value < 0.10 were all entered into the same multivariable models, and backward stepwise eliminations procedures used to retain the variables in the final models as recommended by Collett (20). The performance of the derived model was tested on the development sample and in bootstrap resampling, which was based on 2000 replications. In the bootstrap internal validation based on 2000 replications, the original dataset was resampled at random with replacement 2000 times, with each random sample being of the same size (number of participants) as the original dataset. For each sample, the logistic regressions were fitted, the new beta coefficients estimated and a new equation derived using predictors in the final model. This new model was then tested on the dataset from which it was developed and as well as on the original dataset, and the differences in the performance on the derivation and original samples, were averaged across the 2000 replication to derive the optimism.
The weighting for developed score was derived from regression coefficients and the C-statistic was computed for the correspondent score. The receiver-operating characteristic (ROC) curves were used to derive the optimal cut-off point score, applying the Youden’s index method (21). Performance measures including the sensitivity, specificity, positive and negative predictive values where then estimated at the optimal threshold.
RESULTS
Study Population
During the study period, 513 patients were invited to participate in the study. Seventy six patients were excluded for unavailability of lung function testing and 37 patients for unusable spirometry curves. In total, 400 participants were definitively included in the study. The flow chart of participant’s inclusion is shown on the figure 1.
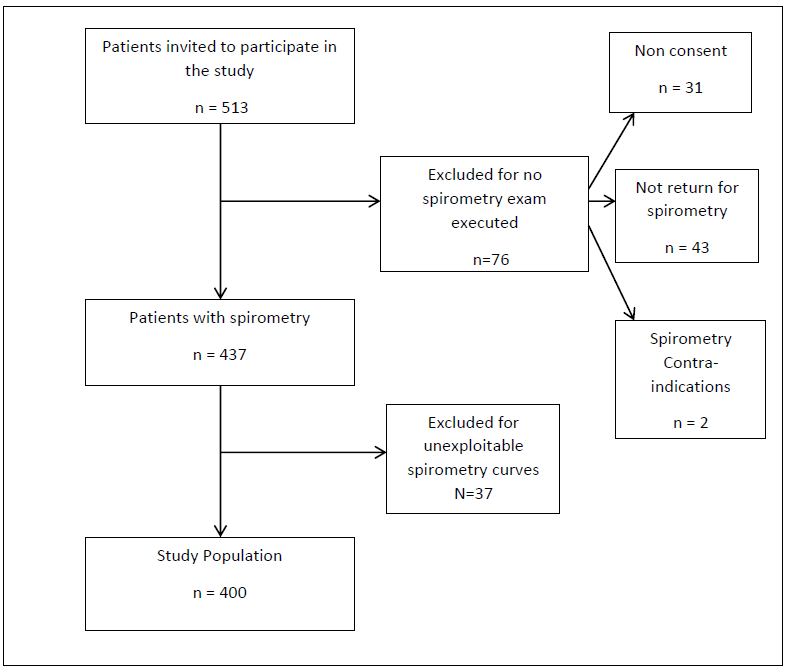
Figure 1: Flow diagram of participant’s inclusion
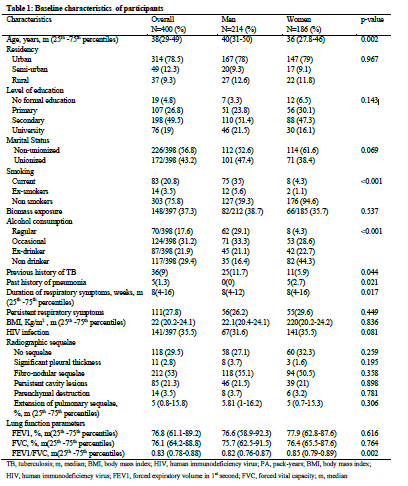
The baseline characteristics of participants are depicted in Table1. There were 214 (53.5%) men and 186 (46.5%) women. The median age (25th -75th percentiles) of men was 40 (31-50) years and that of women was 36 (27.8-46) years (p=0.002). The majority of participants had reached the secondary school level of education (274 participants, 68.5%). In this study, 100 (25%) participants were active smokers and 70 (17.6%) consumed alcohol regularly. The prevalence of smoking was higher in men than in women (35% vs 4.3%, p<0.001). The median (25th -75th percentiles) duration of symptoms before TB treatment was 8 (4-16) weeks in overall group. The prevalence of HIV infection in total sample was 35.5%. In overall sample, 70.5% of the participants had a least one type of radiographic sequelae of TB. Persistent cavity lesions and parenchymal lung destruction were respectively found in 21.3% and 3.5% of participants. The median (25th -75th percentiles) proportion of extension of pulmonary TB sequelae was 5% (0.8-15.8%) in total sample with no significant difference between men and women.
Prevalence and determinants of post-tuberculosis obstructive lung disease
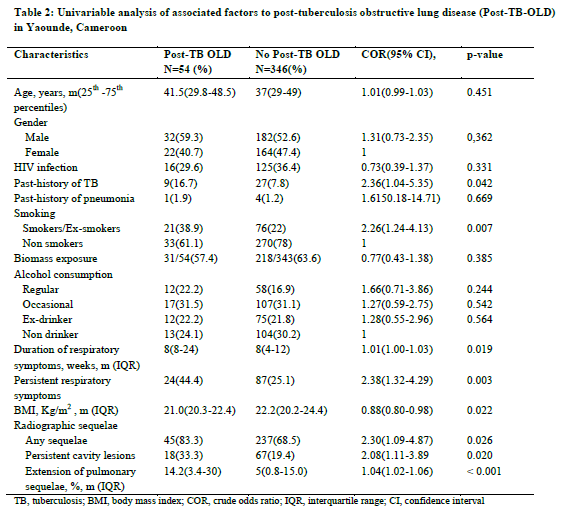
Out of 400 participants, 54 participants had post-TB OLD, giving a prevalence (95% CI) of post-TB OLD of 13.5 % (10.3-17%). The univariable analysis of associated factors to post-TB OLD is depicted in Table 2. Past-history of TB was found in 16.7% patients with post-TB OLD and in 7.8% patients without TB related OLD (p=0.042). Current or past-tobacco smoking was more frequent in patients with post-TB OLD (38.9% vs 22%, p=0.007). Persistent respiratory symptoms at the end of TB treatment and low body mass index were also associated to post-TB OLD. Concerning radiographic features the presence of any TB sequelae, persistent cavity lesions and extension of pulmonary sequelae were significantly associated to post-TB OLD. Indeed, the median (25th -7th percentiles) proportion of pulmonary sequelae was 14.2% (3.4-30%) in post-TB OLD group and 1.04 % (1.02-1.06%) in non-OLD group (p < 0.001).
Prediction score for post-tuberculosis obstructive lung disease
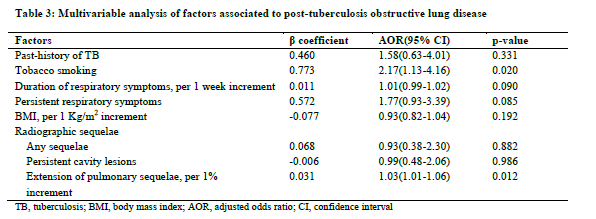
The regression coefficient of 0.783 was higher for history of tobacco smoking than that of the proportion of lung sequelae (0.042), leading to a relative weight of 19 (0.783/0.042) for the presence of history of tobacco smoking. The resulting score for prediction of post-TB OLD (S-TB-OLD) is therefore expressed as: S-TB-OLD= Proportion of lung sequelae (%) + 19 (if tobacco smoking). The discriminant properties of this score are depicted in Figure 2. The AUC was 0.680(0.597-0.763). At the optimal cut-off point score of 19.31, the sensitivity and the specificity were 67% and 65%, respectively. At this cut-off point, the positive predicted value was 22.5% and the negative predicted value was 92.5%.
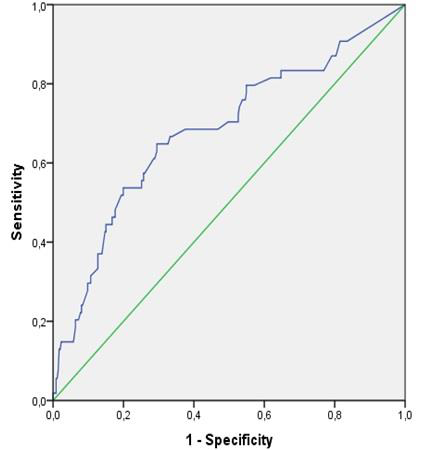
Figure 2: Discrimination curve for post-tuberculosis obstructive lung disease
Models validation
The agreement between the presence of post-TB OLD estimated by the final prediction score and observed post-TB OLD across the continuum of predicted probability was good. The Hosmer and Lemeshow χ2 was 8.135 (p-value=0.321). The discrimination of the model in the derivation sample was good with apparent c-statistic (95% CI) of 0.680(0.597-0.763). The model showed very good stability in bootstrap internal validation method across the 2000 replications with a non-significant optimism at 0.002. The optimism corrected c-statistic was 0.678(0.595-0.761).
Evaluation of risk of post-tuberculosis obstructive lung disease
Based on the prediction score for obstructive lung disease derived above, classification of patients into low risk group (score < 19), moderate risk group (score between 19 and 40) or high risk group (score ≥ 40) for post-TB OLD can be done (Table 4). Class I corresponds to low risk and class III to high risk. As expected, risk of OLD increased across risk classes being 7.5%, 19.8% and 32.4% respectively for class I, II and III.
DISCUSSION
In this study we have found the independent determinants of post-TB OLD and have derived and validated a prediction score for post-TB OLD in adults at the end of treatment of PTB. The
derived model used easily assessable variables in routine clinical practice. Two factors including tobacco smoking and proportion of lung sequelae emerged as the independent associated factors of post-TB OLD. We have also assessed the clinical utility of the derived score (S-TB OLD) and established a classification of patients into risk groups with respect to the score obtained with those with S-TB OLD ≥ 40 at highest risk. The model developed in this study has perfect calibration and good discrimination with only marginal variation during boostrap internal validation.
Tobacco use was associated with post-TB OLD. In fact, smoking has a known deleterious effect on the decline of lung function and is the main risk factor for the occurrence of chronic obstructive pulmonary disease(22). In a study conducted in a low-smoking Indian women population (smoking prevalence at 5% and 73.6% of participants cumulating less than 2.5 pack-years), smoking was associated with lower FEV1(23).
In this study, the radiographic extension of sequelae of TB summarized in terms of the proportion of radiographic sequelae was associated to OLD. Few studies have investigated the association between the extent of initial radiographic lesions or radiographic sequelae and impaired lung function. Radovic et al. On a small sample size (n = 40 patients) showed that initial radiographic extension is associated with low FEV1 and post-tuberculous airflow obstruction(24). These radiological lesions occur because, PTB heals leaving pleuro-parenchymal sequelae of varying degrees due to its fibrogenic character(3–5).
Obstructive post-tuberculous lung disease may be related to bronchial stenosis, diffuse bronchial inflammation, paracicatricial emphysema, or bronchiectasis(25). Progress has been made in understanding the inflammatory mechanisms that are likely responsible for tissue damage and impairment of lung function. Inflammatory mediators involved in cavity formation and airflow obstruction are tumor necrosis factor-α (TNF-α), interleukins 1β, 6, 8, 12, hypoxia inducible factor (HIF), nuclear factor (NF) , matrix metalloproteinases 1, 3, 8, 9,12 and interferon γ. The immune factors associated with fibrosis and restriction include transforming growth factor β (TGF-β), TNF-α, and interleukins-1β, 6, 8, 12 (25). Age, gender, and duration of disease before management do not appear as independent factors associated with OLD in this study, contrary to what Poh reported in 1975 and Lee et al. in Taiwan in 2011(13,26). This could be explained by the fact that our study population was relatively younger than that found in these studies, in particular that carried out by Lee et al. in Korea where the mean age of the population was 52 years.
The model developed in this study was based on two predictors of post-TB OLD including proportion of lung sequelae and smoking. Calibration and discrimination were good. Specifically, the negative predictive value at the threshold score is very high, virtually indicating that subjects with a score <19 will not have post-TB OLD. However, due to the anticipated difference in post-TB OLD prevalence across settings, it is an expectation that the model may require some adjustment where appropriate in order to maintain optimal calibration properties. There are practical examples to support that this adjustment is easily achieved through simple intercept recalibration(27). By using bootstrap resampling for internal validation, we were able to use all participants with valid data for model development, therefore achieving a high number of outcomes per candidate variable tested. This developed score has a number of potential clinical applications including assessment and follow-up of patients with PTB, particularly in low-resources and highly endemic areas for tuberculosis.
The main limitation of this study is related to the lack of data on respiratory function prior to the diagnosis of TB. Furthermore, subjects were recruited within one month of completion of TB treatment. Some studies have shown that the deterioration of lung function worsens over time (10,28) . We cannot analyze the decline in lung function over time due to both the cross-sectional nature of our study and the inclusion of subjects in the month following the end of treatment.
CONCLUSION
The prevalence of post-TB OLD in this study was 13.5%. Tobacco smoking and radiographic extension of sequelae emerged as predictors of post-TB OLD. We have developed a simple numerical score to predict post-TB OLD in high TB endemicity area based solely of these two predictors. The preliminary promising findings from this study require further confirmation through independent external validation studies. Confirmation of the usefulness of this score should help to stratify patients according to the risk of developing post-tuberculous obstructive lung disease in order to improve their management.
Conflicts of interest: None
Author’s contribution:
PYEW conceived the study, supervised data collection, co-analysed the data and drafted of the manuscript; BAD, DA, PMV, MB, KAFG, TB, YCF collected data, contributed to data analysis and critically revised the manuscript, KC and KAP contributed to data analysis, drafting and critical revision of the manuscript. All authors approved the final version of the manuscript.
Acknowledgments: None
REFERENCES
- Organisation mondiale de la santé. QUI | Rapport mondial sur la tuberculose 2018. OMS. Organisation mondiale de la santé; 2018. Disponible sur: http://www.who.int/tb/publications/global_report/en/
- Organisation mondiale de la santé. QUI | Cameroun . QUI. Organisation mondiale de la santé; 2018. Disponible sur: https://www.who.int/countries/cmr/en/
- Al-Hajjaj MS, Joharjy IA. Prédicteurs des séquelles radiologiques de la tuberculose pulmonaire. Acta Radiol (Stockholm, Suède 1987). 2000; 41: 533–7.
- Ramos LMM, Sulmonett N, Ferreira CS, Henriques JF, de Miranda SS. Profil fonctionnel des patients atteints de séquelles de tuberculose dans un hôpital universitaire. J Bras Pneumol Publicacao De Da Soc Bras Pneumol E Tisilogia. 2006; 32: 43-7.
- Tchaou M, Sonhaye L, Kotosso A, Adjenou K, Agoda-Koussema L, N’Timon B, Amadou A, Djagnikpo O .. (premier). Aspects radiographiques des séquelles de la tuberculose chez les personnes vivantes avec le VIH / SIDA à Lomé –Togo. J Fran Viet Pneu. 2012. p. 28–31.
- N’dri K, Ake A AC, Konate I, Konan A, Chiedi AS, Abby B CB. Les aspects radiographiques des séquelles de la tuberculose pulmonaire. Med Afr Noire. 2006; 53: 704–8.
- Vecino M, Pasipanodya JG, Slocum P, Bae S, Munguia G, Miller T, et al. Preuve d’une insuffisance pulmonaire chronique chez les patients traités pour une tuberculose pulmonaire. J Infecter la santé publique. Nov 2011; 4 (5-6): 244-52.
- Pasipanodya JG, Miller TL, Vecino M, Munguia G, Garmon R, Bae S, et al. Insuffisance pulmonaire après tuberculose. Poitrine. 2007 juin; 131: 1817-1824.
- Chushkin MI, Ots ON. Fonction pulmonaire altérée après un traitement contre la tuberculose: la fin de la maladie? J Bras Pneumol. 2017; 43: 38–43.
- Chung KP, Chen JY, Lee CH, Wu HD, Wang JY, Lee LN, et al. Tendances et prédicteurs des changements de la fonction pulmonaire après le traitement de la tuberculose pulmonaire. Cliniques (Sao Paulo). 2011; 66: 549-56.
- Pasipanodya JG, Vecino E, Miller TL, Munguia G, Drewyer G, Fernandez M, et al. Les Blancs non hispaniques présentent un risque plus élevé de troubles pulmonaires dus à la tuberculose pulmonaire. BMC Public Health. 2012; 12: 119.
- Jung JW, Choi JC, Shin JW, Kim JY, Choi BW, Park IW. Affaiblissement pulmonaire chez les survivants de la tuberculose: Enquête nationale coréenne sur l’examen de la santé et de la nutrition 2008-2012. PLoS One. 2015; 10: e0141230.
- Poh S. Obstruction des voies respiratoires chez les patients atteints de tuberculose pulmonaire traitée. Singapore Med J. 1975; 16: 43–6.
- Snider GL, Docteur L, Demas TA, Shaw AR. Maladie obstructive des voies respiratoires chez les patients atteints de tuberculose pulmonaire traitée. Suis Rev Respir Dis. 1971 mai; 103: 625–40.
- Organisation mondiale de la santé. Lignes directrices sur le traitement de la tuberculose.Genève: Organisation mondiale de la santé; 2009. 2009; Disponible sur: https://www.who.int/tb/publications/2010/9789241547833/en/
- Mahler DA, Wells CK. Évaluation des méthodes cliniques pour évaluer la dyspnée. Poitrine. 1988; 93: 580-5.
- Ralph AP, Ardian M, Wiguna A, Maguire GP, Becker NG, Drogumuller G, et al. Un score numérique simple et valide pour évaluer la gravité de la radiographie pulmonaire dans la tuberculose pulmonaire à frottis positif adulte. Thorax. Octobre 2010; 65: 863–9.
- Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardisation de la spirométrie. Eur Respir J. 2005; 26: 319–38.
- Quanjer PH, Stanojevic S, Cole TJ, Baur X, Hall GL, Culver BH, et al. Valeurs de référence multiethniques pour la spirométrie pour la tranche d’âge de 3 à 95 ans: les équations de la fonction pulmonaire globale 2012. Eur Respir J. 2012; 40: 1324–43.
- Collett D. Modélisation des données binaires, 2e éd. New York: Chapman & Hall / CRC. 2003.
- Youden WJ. Index pour l’évaluation des tests de diagnostic. Cancer. 1950; 3: 32-5.
- Vijayan VK. Maladie pulmonaire obstructive chronique. Indian J Med Res. 2013; 137: 251–69.
- Arora S, Rasania S, Bachani D, Gandhi A, Chhabra S.Pollution atmosphérique et facteurs de risque environnementaux pour une altération de la fonction pulmonaire chez les femmes adultes d’un bidonville urbain de Delhi: une étude de prévalence. Inde pulmonaire. 2018; 35: 193.
- Radovic M, Ristic L, Ciric Z, Dinic-Radovic V, Stankovic I, Pejcic T, et al. Changements dans la déficience de la fonction respiratoire après le traitement d’une tuberculose pulmonaire sévère – limitations pour la détection sous-jacente de la MPOC. Int J Chron Obstruct Pulmon Dis. 2016; 11: 1307–16.
- Ravimohan S, Kornfeld H, Weissman D, Bisson GP. Tuberculose et lésions pulmonaires: de l’épidémiologie à la physiopathologie. Eur Respir Rev.2018; 27 (147).
- Lee EJ, Lee SY, In KH, Yoo SH, Choi EJ, Oh YW, et al. Un test de fonction pulmonaire de routine peut estimer l’étendue du poumon tuberculeux détruit. ScientificWorldJournal. 2012; 2012: 835031. UNE
- Kengne AP, Beulens JWJ, Peelen LM, Moons KGM, van der Schouw YT, Schulze MB, et al. Scores de risque non invasifs pour la prédiction du diabète de type 2 (EPIC-InterAct): une validation des modèles existants. lancet Diabetes Endocrinol. 2014; 2: 19-29.
- Lee SH, Kwon AM, Yang HC, Lee SK, Kim Y, Choi JH et al. Diminution de la fonction pulmonaire longitudinale chez les sujets atteints de tuberculose pulmonaire guérie spontanée. PLoS One. 2016; 11: e0164039.
